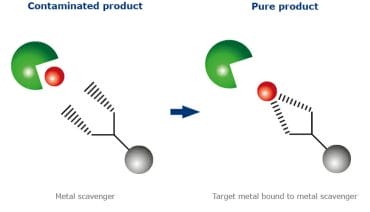
-
Meaning of Scavenger in chemistry:
– like the carbohydrazides used in Electron Microscopy (purifies or traps the products of impurities or free radicals.
Wikipedia sources with our modifications
A chemical scavenger is a chemical substance added to a mixture to remove or deactivate impurities and unwanted reaction products, for example oxygen, to ensure that they will not cause any adverse reaction. Their use is varied:
In atmospheric chemistry, the most common trap is the hydroxyl radical, a short-lived radical produced by photolysis in the atmosphere. It is the most important oxidant for carbon monoxide, methane and other hydrocarbons, sulfur dioxide, hydrogen sulfide and most other contaminants, removing them from the atmosphere.
In the separation of isotopes by molecular laser, methane is used as a scavenging gas for fluorine atoms.
Hydrazine and ascorbic acid are used as oxygen scavenging corrosion inhibitors.
Tocopherol and naringenin are bioactive free radical scavengers which act as antioxidants; synthetic catalytic sensors are their synthetic equivalents
Organotin compounds are used in the manufacture of polymers as hydrochloric acid scavengers.
Oxygen scavengers or oxygen scavengers are small self-adhesive pouches or labels that are placed inside modified atmosphere packages to help extend the shelf life of the product (especially cooked meats) and improve the appearance of the product. They work by absorbing any oxygen left in the packaging by oxidizing the iron powder in the sachet / label. [1]
Glutathione in the body removes oxidizing free radicals and peroxides and, as a nucleophilic thiol, attacks dangerous alkylating electrophiles, which can be exogenous toxins or produced during metabolism (eg NAPQI from paracetamol).
^ Https://www.newscientist.com/article/mg18224443.600-wrappers-smarten-up-to-protect-food.html New Scientist, April 24, 20042004