this contribution by By Eric Hand
The Laboratory for Molecular Biology (LMB), clad in glass the color of sea ice, rises like a futuristic factory above the rapeseed fields of Cambridge, U.K. It is the crown jewel of the U.K. Medical Research Council, a storied government lab that has garnered more than a dozen Nobel Prizes. One of the first came in 1962 after LMB researchers, having pioneered x-ray crystallography, used the technique to decipher the first atomic structures for proteins—those of myoglobin and hemoglobin, which carry oxygen in muscle tissue and blood. X-ray crystallography has dominated structural biology ever since, but it has an Achilles’ heel: Some proteins just can’t be coaxed to form crystals, which scatter x-rays to reveal structure.
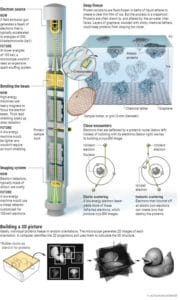
One of LMB’s most recent Nobel laureates is Richard Henderson, an unassuming Scotsman who slouches in his office chair in socks and sandals, surrounded by mountains of paperwork. In 2017, Henderson won a share of the chemistry prize for his work in developing detectors for cryo–electron microscopy (cryo-EM). The technique, also pioneered at LMB, is the brash upstart in structural biology, challenging crystallography in resolution and surpassing it in purview: It opens up far more proteins to inspection and captures many more of their natural configurations.
Cryo-EM dodges the problem of crystallization with a flash-freezing process that fixes proteins in thin films of glassy ice. Then, an electron microscope takes thousands of 2D snapshots of the proteins caught in random orientations. A computer stitches them together to reveal the 3D structure, so important in understanding how a protein works—and how a drugmaker might target it
But cryo-EM has a big problem of its own: long waits to use extraordinarily expensive microscopes. Moreover, tricky sample preparation means that even when researchers get access, much of their time ends up wasted. “There’s this dirty little secret of the field,” says LMB physicist Chris Russo. “It’s almost as hard as making a crystal. There’s a lot of trial and error in it.”
Some researchers are aiming to fix those problems with automation: robots that can make icy protein samples more reliably and with less waste. Other scientists are developing materials that protect protein molecules during freezing. Still others are working on software that could gather data more efficiently.
But Henderson sees those efforts as mere Band-Aids. For him, what holds back the technique is the forbidding cost of a microscope. Henderson, Russo, and a small group of confederates are trying to make cryo-EM affordable.
A top machine costs about $7 million. Preparing a room and installing a microscope can cost just as much. Then come the operational costs—a torrent of electricity, dedicated troubleshooting staff—that can rise to $10,000 per day.
Roughly 130 Krios machines—the microscopes widely considered the best—have been sold by Thermo Fisher Scientific and installed around the world. LMB has the luxury of three for a relatively small staff, and yet even its researchers must wait a month or more to get time.
Most structural biologists have no access at all. “The wait can be from 3 months to infinity,” says Bridget Carragher, codirector of the Simons Electron Microscopy Center in New York City, a mecca for cryo-EM. “It’s becoming the haves and the have-nots.”
No one complains about the quality of the Krios machines, which take months to assemble by hand from thousands of parts in a Dutch factory. They are Cadillacs. But science would benefit from something less posh, Henderson says. “We need a people’s cryo-EM for maybe 10 times less: a Volkswagen Beetle.”